What Is a Structured Benefit-Risk Framework (SBRF)? And Why the BRAD Should Be a Living Document
In biotech, product development is full of trade-offs. How much benefit does a treatment provide? What are the associated risks? And how do you explain those trade-offs—clearly and consistently—to regulators, investors, or even your internal team?
That’s where the Structured Benefit-Risk Framework (SBRF) comes in. Recommended in the CIOMS XII report [1], it’s designed to support clear, structured decision-making throughout the lifecycle of a medicinal product.
At the center of this approach is the Benefit-Risk Assessment Document (BRAD), a dynamic working document that reflects your current thinking as new data and context emerge.
What’s In A Structured Benefit-Risk Framework?
The SBRF provides a consistent structure for evaluating and communicating benefit-risk trade-offs. It includes:
Therapeutic context - What medical need are you addressing, and what does the current treatment landscape look like?
Key benefits - Expected improvements in clinical outcomes, patient function, quality of life, etc.
Key risks - Known and potential adverse effects, tolerability issues, and long-term safety considerations.
Risk minimization strategies - Monitoring, labeling, and other actions to mitigate risk.
Uncertainties - Gaps in knowledge—what’s still evolving or unknown.
Overall trade-off assessment - A concise summary of whether the benefits outweigh the risks, and under what conditions.
This framework is increasingly recognized by global regulatory bodies as best practice [3, 5].
What Is The BRAD, And Why It Shouldn’t Be Static
The BRAD is where benefit-risk thinking gets documented in a structured, traceable way. While it's essential for regulatory submissions, it also serves as a practical tool to support internal decision-making and maintain alignment as your program develops.
A 2023 case study from Roche showed how building a structured, evolving BRAD helped align cross-functional teams throughout development [2]. When used intentionally, the BRAD becomes a consistent reference point—even as people, data, and priorities shift.
Teams that treat the BRAD as a static output miss the bigger opportunity: it’s a tool for insight, transparency, and continuity.
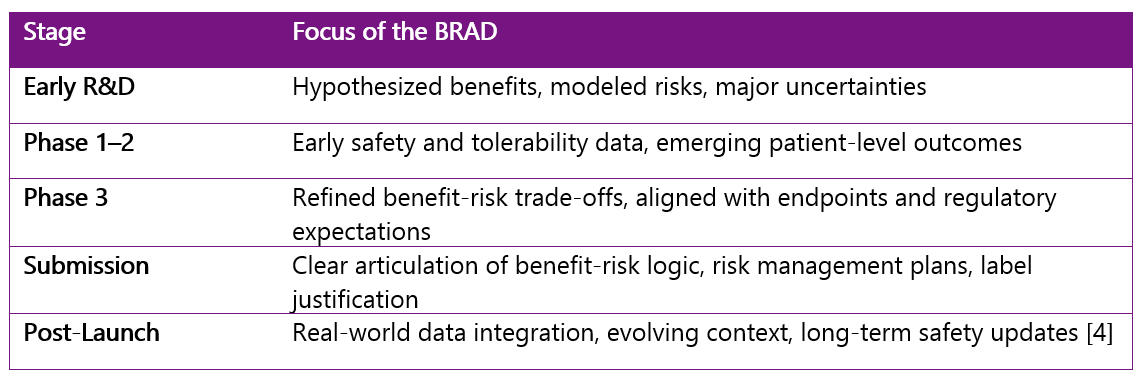
How The BRAD Evolves Over Time
One of the BRAD’s most important strengths is its adaptability. It should grow and change alongside your program—capturing new insights, data, and context at each stage of development. Here's a look at how the focus of the BRAD typically shifts across the product lifecycle:
Keeping the BRAD active through each of these stages builds continuity across teams, reduces duplication of effort, and ensures that key decisions remain traceable and transparent. It’s a working document—one that should reflect not just current data, but also how understanding and priorities develop over time.
Visualization Tools: Making The Trade-Offs Clear
Visuals like value trees, forest plots, and benefit-risk tables can help translate complex evaluations into formats that support clearer decision-making.
These tools are especially effective when preparing for regulatory interactions, go/no-go meetings, or investor updates. They're also helpful when patient and clinical priorities differ, and structured communication is key.
Why This Matters For Biotech Teams
For smaller or resource-constrained biotech teams, the SBRF offers a grounded framework that supports consistency, decision clarity, and cross-functional alignment without adding unnecessary complexity.
Here’s how teams can benefit from applying the SBRF early and revisiting it often:
Helps align clinical, regulatory, safety, and commercial teams
Builds investor and partner confidence by showing strategic clarity
Tracks evolving assumptions and decisions in a consistent way
Prevents knowledge loss during handoffs or organizational shifts
How To Start Applying The SBRF
Draft a basic value tree and capture your early thinking on key benefits and risks
Create a simple BRAD shell with your clinical and regulatory leads
Update it consistently, especially after new data readouts or milestone reviews
Use it actively and bring it into team meetings and cross-functional planning, not just submission prep
Final Thoughts
Used intentionally, the Structured Benefit-Risk Framework helps teams clarify trade-offs, align across functions, and build a clear record of how key decisions evolve over time. When maintained intentionally, the BRAD provides a structured record of benefit-risk thinking that supports both internal decision-making and external communication.
For early-stage biotech teams, this approach provides needed structure without overcomplicating decision-making. It helps reduce uncertainty, preserve institutional knowledge, and support communication—both internally and externally—as programs evolve and stakeholders shift.
If you’re navigating a high-uncertainty program, managing limited resources, or preparing for conversations with regulators or investors, the SBRF can be your anchor and your advantage.
Start small, build consistently, and let your BRAD reflect the thinking behind your science—not just the outcomes.
References
1. CIOMS Working Group XII. Benefit-Risk Balance for Medicinal Products. Geneva: Council for International Organizations of Medical Sciences (CIOMS), 2025. https://doi.org/10.56759/gwfz1791
2. Wehrli T, Hemmings R, McAuslane N, et al. A Structured Benefit‑Risk Assessment Operating Model for Investigational Medicinal Products. Therapeutic Innovation & Regulatory Science. 2023. https://link.springer.com/article/10.1007/s43441-023-00508-2
3. Regulatory Affairs Professionals Society (RAPS). Regulators, Industry Tout New Approach for Benefit-Risk Assessments. February 2024. https://www.raps.org/news-and-articles/news-articles/2024/2/regulators%2C-industry-tout-new-approach-for-benefit
4. Kohl KS, Kochhar S, Nandy R, et al. Benefit-Risk Assessment of Vaccines. Vaccine. 2023;41(34):5103–5110. https://www.sciencedirect.com/science/article/pii/S0264410X23008721
5. DIA Global. Session 7: Insights on Benefit-Risk Assessment Across the Product Lifecycle. Global Pharmacovigilance and Risk Management Strategies Conference. 2024. https://www.diaglobal.org/en/conference-listing/meetings/2024/02/global-pharmacovigilance-and-risk-management-strategies-conference/agenda/06/session-7-insights-on-benefit-risk-assessment